Vo Van Nhan, DDS, PhD1, Le Van Son, DDS, PhD2,
Ta Anh Tuan, DDS, PhD3, Nguyen Tai Son, DDS, PhD4,
Trinh Dinh Hai, DDS, PhD5, Le Duc Lanh, DDS, PhD6,
Nguyen Manh Ha, DDS7, and Lam Dai Phong, DDS, PhD
Link gốc bài viết: https://pubmed.ncbi.nlm.nih.gov/29351041/
Abstract
Objective:
To evaluate 2 iliac corticocancellous-block grafting techniques for dental implant placement in residual alveolar clefts.
Design:
Nonrandomized prospective clinical trial between March 2010 and December 2014.
Setting:
National Hospital of Odonto-Stomatology, Hanoi, Vietnam.
Participants:
Thirty-two patients (23 female, 9 male; mean age, 21.28 years; range, 16-31 years) with unilateral complete alveolar cleft after reconstructive surgery for cleft lip and palate (CLP).
Interventions:
Harvested iliac crest bone was cut into 2 corticocancellous blocks. The smaller block was adapted against the sutured nasal mucoperiosteum and overlaid with cancellous bone; the larger one overlapped the labial cleft margin and was fixed with screws. Endosteal dental implants were placed after 4 to 6 months, and final restorations were delivered 6 months later.
Main Outcome Measures:
Flap statuses were assessed clinically. Bone formation was assessed using the Enemark scale. Cone-beam computed tomography was used for graft height and width measurements. Implant health was assessed by the Misch criteria.
Results:
The mean postgrafting follow-up period was 36.7 ± 10.4 (range, 18-53) months. Three patients (9.4%) showed flap dehiscence but no infection 7 days after bone grafting. Twenty-nine patients (90.6%) had 75% to 100% bone fill (Enemark score of 1). The mean graft height and width were 11.4 ± 2.4 and 6.1 ± 1.0 mm, respectively. Sufficient bone for implant placement was noted in 29 patients (90.6%); the others required partially fixed prostheses. All implants functioned for at least 18 months.
Conclusion:
The proposed technique is reliable to reconstruct the alveolar cleft for implant placement in CLP patients.
Keywords
secondary bone graft, dental implant
Introduction
Cleft lip and palate (CLP) management is a long process that can begin prenatally and continue into adulthood (Posnick and Ruiz, 2002). The multidisciplinary approach includes primary CLP repair, alveolar bone grafting, orthodontic treatment, and prosthetic rehabilitation. Endosteal dental implants are a viable option for prosthetic rehabilitation of patients with alveolar cleft and congenitally missing teeth (Verdi et al., 1991). They provide satisfactory functional and aesthetic outcomes and avoid the disadvantages of prosthodontic treatments. However, they require adequate bone volume and quality for implant survival. Alveolar bone grafting, introduced by Boyne and Sand (1972), is a standard procedure for most patients. Its aims are to (1) allow spontaneous eruption or orthodontic movement of the canine or lateral incisor into the cleft area (Dempf et al. 2002; Takahashi et al., 2011); (2) maintain bony support of teeth adjacent to the cleft and prevent collapse of alveolar segments (Cho-Lee et al., 2013); (3) enable oronasal fistula closure (Cho-Lee et al., 2013; Takahashi et al., 2011); (4) support the alar base and nose (Cho-Lee et al., 2013); (5) improve speech, articulation, and nasality (Bureau et al., 2001); and (6) facilitate dental implant placement (Takahashi et al., 2011). The reported techniques include autogenous cancellous bone grafted with a sutured nasal flap (Feichtinger et al., 2007; Kindelan et al., 1997; Long et al., 2000), pyramidal iliac corticocancellous block grafted with the cortical surface contacting the nasal mucosa and interspaced with cancellous chips (Cho-Lee et al., 2013), autogenous iliac particulate bone grafted along with a resorbable or nonresorbable membrane (Peled et al., 2005) or titanium mesh and screw fixation (Takahashi et al., 2011), iliac cortex bone plate grafted into the palatal deficiency and particulate marrow and cancellous bone (PMCB) packed between the cortical bone and the reconstructed nasal flap (Ishii et al., 2002), and 2 lateral cortical bone plates from the mandibular symphysis grafted against the labial and palatal cleft margins without screw fixation or intervening particulate bone (Mikoya et al., 2010).
A new technique involves grafting of 2 iliac corticocancellous blocks with interspaced cancellous bone. It combines the advantages of the quick healing of cancellous bone and the strong mechanical properties of cortical bone and can be applied to defects of any size. The purpose of this nonrandomized prospective clinical study was to evaluate the suitability of the iliac corticocancellous-block grafting technique for dental implant placement in residual alveolar clefts.
Materials and Methods
Ethical Approval
Details of the study and procedures were explained to each patient, and their written consents were obtained. The experimental protocol was approved by the Institutional Ethics Committee.
Patients
The subjects were 32 patients (23 female and 9 male) who had undergone reconstructive surgery for CLP. The inclusion criteria were age over 16 years and the presence of residual unilateral complete alveolar cleft with or without oronasal fistula. Patients with bilateral incomplete alveolar cleft and those who had not yet undergone reconstructive surgery for CLP were excluded.
All patients were treated according to the same protocol (Figure 1). Before alveolar bone grafting, orthodontic leveling, alignment, and expansion of the maxillary arch were performed to create a mesiodistal space suitable for the final restoration.
Figure 1. Flow diagram of the study.
Bone Harvesting
The hip was elevated on several rolled towels. Under general endotracheal anesthesia, the location of the anterosuperior iliac spine was marked and the skin was incised beginning 1 cm posterior to this landmark, to avoid damaging the lateral femoral cutaneous nerve. The incision was for approximately 5 cm parallel to the iliac crest and oriented toward the posterosuperior iliac spine. The iliac crest was palpated, and its margin was exposed by cutting the fascia and periosteum. Using a medial approach, the monocortical bone was cut with a piezotome ultrasonic device and removed with a chisel. The cancellous bone was harvested with curettes, as needed. A hemostatic sponge was placed, and the wound was closed in 2 layers with periosteal and intradermal sutures of coated Vicryl 4.0 (Polyglactin 910; Ethicon, Somerville, NJ).
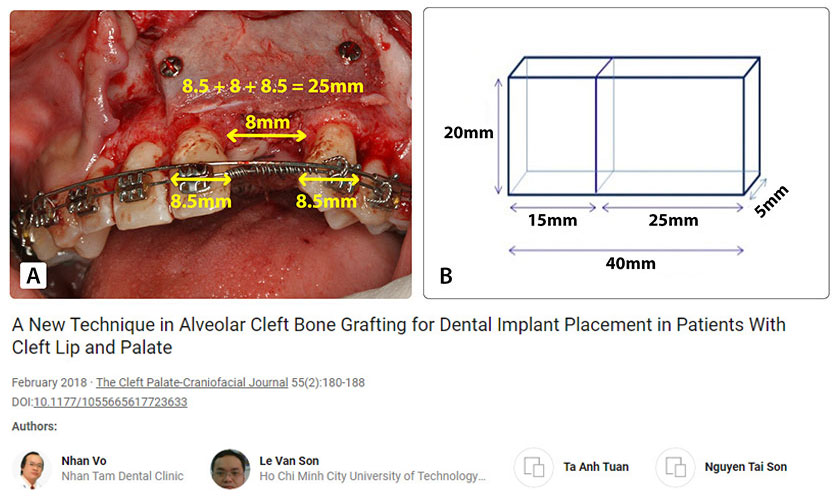
By summing the mean widths of the central incisor (∼8.5 mm), lateral incisor (∼8 mm), and canine (∼8.5 mm), the required labial block size was estimated as 25 mm × 20 mm × 5 mm (Figure 2A). The palatal block size was 15 mm × 20 mm × 5 mm because an alveolar cleft is usually wider at the nasal floor. Therefore, the total size of the harvested bone was 40 mm × 20 mm × 5 mm (Figure 2B).
Figure 2. Bone harvesting technique at the iliac crest. (A) The size of the labial bone block. (B) The total size of the bone block harvested from the iliac crest.
Alveolar Bone Grafting
Alveolar bone grafting was performed by the same experienced oral-maxillofacial surgeons. The recipient site was anesthetized by infiltration of lidocaine with 1:200,000 epinephrine. The incision was begun along the cleft margin from the labial to the palate sides. The labial sulcular incision was carried along 2 or 3 adjacent teeth; then, 2 vertical relieving incisions were made and curved (120°) anterosuperiorly at the ends. The palatal sulcular incision was continued to the second premolar, and a vertical releasing incision was made from this area toward the palatal midline, allowing anterior advancement of the palatal attached mucosa (Amin et al., 2002). Full-thickness labial and palatal flaps were reflected. The labial flaps had periosteal releasing incisions, ensuring an adequate nasal lining for watertight closure and sufficient sliding of the flaps. The harvested bone was cut into 2 corticocancellous blocks. The smaller block (palatal block) was shaped to fit the defect margin nasally and inserted against the sutured nasal lining, with the cortical bone layer toward the palatal side (Figure 3A, 3B). The cleft was then nearly filled with harvested particulate cancellous bone.
Figure 3. Bone grafting technique. (A) The alveolar cleft on CT Cone Beam. (B) Size of the alveolar defect (yellow line). (C) The technique of two iliac corticocancellous-block grafting.
The larger block (labial block) was placed on the particulate cancellous bone, with the cortical bone layer toward the labial side and fixed with screws. It covered the radicular bone of the 2 teeth adjacent to the cleft and extended from the alveolar crest to the nasal floor (Figure 3C). During screw fixation, finger pressure was used to protect the palatal flap.
The labial flaps were approximated and closed with interrupted sutures. The palatal and labial mucoperiosteum was closed with horizontal mattress and interrupted sutures. As all the flaps were advanced, keratinized tissue overlaid the alveolar ridge and tension-free closure was achieved.
All patients received a preoperative intravenous antibiotic (1 g ceftriaxone) and oral antibiotics (1 million units of penicillin G or 300 mg clindamycin if allergic to penicillin) for 10 days postoperatively. The patients were hospitalized for 1 day to allow observation.
Implant Surgery and Final Restoration
After 4 to 6 months, an endosteal dental implant was placed in the grafted site using an acrylic surgical stent. The outpatient procedure was performed under local anesthesia with undersized site preparation to achieve primary implant stability. Additional bone was grafted simultaneously with implant placement by the guided bone regeneration (GBR) or bone ring technique (large defects). For GBR grafting technique, we used a mixture of autogenous particulate bone and Osteon (30% hydroxyapatite and 70% β-tricalcium phosphate) with resorbable membrane (Genoss, Suwon, Korea). Autogenous particulate bone is made from autogenous bone harvested at the position of the implant bed by a 3-mm-diameter trephine bur. Bone ring grafting is a 3-dimensional crestal bone augmentation technique for vertical defect. The bone ring was harvested from symphysis or retromolar in a manner similar to that of Stevens (2010).
Six months thereafter, a healing screw was placed. Three weeks later, impressions were made for the final restoration.
Outcome Measures
The following parameters were evaluated 7 days and 4 to 6, 12, 18, 24, and 36 months after alveolar bone grafting: (1) flap status (dehiscence and infection), (2) fistula status, (3) bone formation, and (4) implant health.
The scale described by Enemark et al. (1987) was used to evaluate bone bridging radiographically: scores 1, 2, 3, and 4 represented 75% to 100%, 50% to 75%, 25% to 50%, and less 25% bone fill in the grafted site, respectively. Scores 1 and 2 were considered successful, score 3 suggested partial failure, and score 4 indicated complete failure.
A radiopaque marker (Gutta-percha) was placed vertically along the potential axis of the final restoration, and cone-beam computed tomography (CBCT) scans were obtained with the surgical stent in place. The images were imported into an imaging program (EasyDent V4 Viewer; Vatech, Suwon, Korea) to measure graft dimensions suitable for implant placement. Height was recorded from the lowest point to the highest point of the graft (d in Figure 4). Width was calculated by averaging the buccolingual measurements of the coronal, middle, and apical one-thirds of the graft (c, b, and a, respectively, in Figure 4). Assuming that standard implants of 10 mm length would be biomechanically sound in the lateral incisor region, grafted bone of at least 7 mm height and 4 mm width was required for implant placement. Implant health was evaluated by the Misch et al. (2008) criteria through 4 clinical groups. The successful and satisfactory survival groups showed no pain or tenderness during function, no mobility, and no history of exudate; on radiographs, the crestal bone showed a loss of <2.0 mm in the former group and from 2 to 4 mm in the latter group. However, the group with compromised survival may have sensitivity and history of exudates, but no mobility; on radiographs, this group showed bone loss >4 mm (less than half the length of the implant). In the failure group, all of the following were observed: pain during function, mobility, uncontrolled exudate, and radiographic bone loss more than half the length of the implant or loss of the implant.
Figure 4. Radiographic assessment of grafted bone: height and width measurements on an axial computed tomographic slice.
Statistical Analysis
Imaging data were scored independently by 2 experienced observers (17 years’ experience and 11 years’ experience) who were blinded to the patients. The assessments were repeated approximately 2 weeks later. The agreement between repeated measurements by the same observer at different time points and between the observers at each time point was analyzed using unweighted kappa statistics. Proportions were compared by chi-square tests using SPSS 16.0 software (SPSS, Inc, Chicago, IL).
Results
The mean age at the time of alveolar bone grafting was 21.28 years (range, 16-31 years). The mean follow-up period was 36.7 months (range, 18-53 months); further, 29 and 20 patients were followed for more than 18 and up to 36 months, respectively.
Seven days postoperatively, complete closure of the oronasal fistula was achieved in all 32 patients. Flap dehiscence was observed in 3 patients (9.4%), resulting in grafted bone exposure; however, the wound closed after exfoliation of the small bone fragments. At 4 to 6 months, all patients had good mucosal healing.
At 4 to 6 months postoperatively, 29 patients (90.6%) had an Enemark score of 1, and 3 patients (9.4%) had a score of 3. On CT Cone Beam, the mean height and width of the grafted bone evaluated on 32 patients were 11.4 ± 2.4 and 6.1 ± 1.0 mm, respectively. In 3 cases (9.4%), the graft height was 4.1 to 4.7 mm and the width was 3.6 to 3.8 mm that was insufficient bone for implant placement. Those 3 patients were indicated conventional fixed dental prostheses. As a result, they were not enrolled in the evaluation of bone around implant in the study. The rest of the 29 patients who had sufficient bone for implant placement was continued to conduct the implant procedure. A significantly high number of patients (90.6%) had sufficient bone (>10 mm height and >5 mm width) for implant placement (P < .05).
Therefore, the number of patients who remained in further evaluation process was 29. Thirty-two implants with a resorbable blast media surface were placed (3 patients received 2 implants each). A fixture diameter of 3.8 mm was chosen after clinical assessment of the alveolar bone width. A fixture length of either 10 mm (31 cases) or 12 mm (1 case) was selected by determining the height of the cleft site. Insertion torque over 20 N/cm2 was used in 56.2% of the cases.
Additional bone grafting was performed simultaneously with the implant placement in all of 29 patients for supporting the soft tissue and improving the aesthetics of the prosthesis. GBR grafting technique and bone ring technique was used. Among the 32 implants placed in our study, GBR grafting technique was indicated for 29 because of small defect size. Another 3 implants had greater bone defect, which required bone support around the crestal implant and was accomplished by a bone ring technique, with the bone harvested from the retromolar in 2 patients and from the symphysis in 1 patient. One patient who had 2 implants placed underwent GBR grafting in 1 implant and bone ring in another at the same time.
At 12 months after alveolar bone grafting, all of 29 patients were assigned an Enermark score of 1. Based on the Misch criteria, 100% of the implants were successful.
At 18 months, one implant showed 2 mm marginal bone loss, so the Enermark score was changed from 1 to 2. That implant had satisfactory survival. However, all the implants survived during function; that is, 31 of the 32 implants (96.9%) were successful, and 1 implant (3.1%) survived satisfactorily.
After 36 months of follow-up, 20 patients with 23 implants were followed up; 22 of 23 implants (95.7%) were successful (Enermark score of 1), and 1 implant (4.3%) had satisfactory survival (Enermark score of 2). No significant differences in the amount of bone fill and the survival rates were noted between the time points (18, 24, and 36 months) (P > .05) (Table 1).
Table 1. Results of Bone Formation.
Discussion
Choice of Donor Site
Despite the various donor sites described in the literature, including the tibia (Chen et al., 2006), rib, calvarium (Amanat and Langdon, 1991; Cohen et al., 1991; Denny et al., 1999; Kline and Wolfe, 1995), and mandibular symphysis (Booij et al., 2005; Bukhari et al., 2009; Mikoya et al., 2010), the iliac crest is the most preferred (Canady et al., 1993; Murthy and Lehman, 2005). In a survey of 110 centers with 240 American Cleft Palate-Craniofacial Association teams, Murthy and Lehman (2005) reported that 92 (83%), 9 (8%), 3 (2.7%), 2 (1.8%), and 5 (4.5%) centers used the iliac crest, calvarium, rib, tibia, and other bones as donor sites for alveolar bone grafting (Murthy and Lehman, 2005). Iliac cancellous bone, with osteogenic cells, produces rapid vascularization of the graft but undergoes greater resorption (Albrektsson, 1980; Swan and Goodacre, 2006) than iliac cortical bone. The main patient complaints are gait disturbance, prolonged recovery, and hospitalization (Swan and Goodacre, 2006), but these complications are present for only a short time (Canady et al., 1993). Additionally, several authors have been studying materials to replace autogenous bone in alveolar bone grafting, such as demineralized allogeneic bone matrix mixed with bone marrow (Goudy et al., 2009), microstructured β-tricalcium phosphate (de Ruiter et al., 2015), and bone morphogenetic protein-2 (Dickinson et al., 2008). However, studies of these materials in patients with CLP are limited, and so far these materials commonly have not been used in a clinical setting. Therefore, autogenous bone from the iliac crest is still considered the gold standard. The iliac crest was chosen in the present study because it provides the large bone volume required for the proposed technique.
Grafting Technique
Our technique of 2 iliac corticocancellous blocks grafting obtained some advantages compared to reported techniques. The 2 iliac corticocancellous block grafting involves the palatal block that is adapted to the cleft margin in the palate; especially, the labial block covers the cleft margin with rigid screw fixation of the block grafts, which is not only considered as a key factor in graft success (Bosshardt and Schenk, 2009) but also acts as a barrier to prevent soft tissue migration into the graft and provides a favorable environment for bone formation, and no membrane is required during bone grafting. Besides, consolidation is improved by packing particulate cancellous bone tightly between the blocks, providing ideal osseous support for dental implants. One of the other advantages of our technique is that the cancellous part allows rapid vascularization and healing (Albrektsson, 1980), and the mechanical properties of the cortical part limit resorption and facilitate primary implant stability. Additionally, our technique provides adequate bone around the neck and apex of the implant and supports the alar base and nose because the graft spans from the alveolar crest to the nasal floor. Moreover, both of the blocks were adapted to the cleft quickly, which shortens the operative time.
The flap design influences the success of not only the bone graft but also the dental implant. The flaps should provide maximum attached mucosa near the alveolar crest and around the implant neck to limit recession and ensure the long-term success of the implant (Kazemi et al., 2002). In this study, a combination of lateral sliding flaps with periosteal release and a palatal flap was used. The flaps have excellent blood supply, ensure adequate attached gingiva near the alveolar cleft and tension-free closure, and reduce the risk of dehiscence. Complete closure of the oronasal fistula was achieved in all patients, and only 3 patients showed flap dehiscence. The dehiscence rate (9.4%) was much lower than that reported by Peled et al. (2005) (40%) or Denny et al. (1999) (17%). This complication can be explained by the significantly larger bone volume placed labially to overlap the defect margin, compared with grafting of cancellous bone or a bone block only up to the cleft margin (Cho-Lee et al., 2013; Feichtinger et al., 2007), potentially increasing flap tension and leading to dehiscence. In cases of wide defects, a thinner grafted-block is recommended first, and additional bone graft is performed during implant surgery.
Orthodontic Treatment
Most authors prefer proper presurgical expansion of the maxillary arch because it results in less resistance, greater access for oronasal fistula closure and bone grafts, better postoperative hygiene, and a lower chance of recurrence of the fistula. In tertiary bone grafting, the main advantage of presurgical orthodontic treatment is the creation of a suitable horizontal space for implant placement (Figure 5A). It facilitates designing and determination of the graft volume as well as planning of the location and orientation of the dental implant.
Figure 5. Postoperative orthodontic treatment. (A) Presurgical orthodontic treatment. (B) Black triangles adjacent to the final restoration. (C) Orthodontic closure of black triangles.
In patients with CLP, the adjacent teeth usually tip into the alveolar cleft, creating black triangles after the final restoration (Figure 5B). Postoperative orthodontic treatment is essential to align the roots and shift the contact points apically for eliminating such gaps (Figure 5C) and improving aesthetics. Furthermore, it places traction on the graft and can stimulate bone formation (Turvey et al., 1984).
Procedural Timing
In patients with alveolar clefts, the interval between alveolar bone grafting and implant placement is of considerable importance. The longer the interval, the greater is the likelihood of alveolar bone resorption (Feichtinger et al., 2007; Kearns et al., 1997; Takahashi et al., 1999). According to Takahashi et al. (1999), the interdental alveolar crest level (IACL) significantly decreases 24 months after bone grafting: they reported that 80% of the grafted alveoli were suitable to place implants within 2 years, but only 44% were adequate after more than 2 years. Kearns et al. (1997) suggested a maximum interval of 4 to 6 months following closure of oronasal fistula and alveolar bone grafting in the permanent dentition, whereas Boyne (1991) advised a 3- to 4-month interval in noncleft patients. In our study, bone loss initially occurred 6 months after bone grafting, around the time of implant placement, in some cases. Therefore, the waiting time was shortened and dental implants were placed after 4 months. In fact, at this time, the border between the grafted bone and the native bone was indistinct, confirming good healing.
The IACL also seems to be influenced by patient age during bone grafting. Secondary bone grafting of alveolar clefts in patients with CLP should be performed before canine eruption because of the better clinical results and greater osteogenic activity in younger patients (Dempf et al., 2002; Denny et al., 1999; Takahashi, 2011). Åbyholm et al. (1981) found a higher failure rate when osteoplasty was performed late. Yet, implant placement is generally not recommended before the growth spurt (Lekholm, 1993) because implants, similar to ankylosed teeth, do not move along with other parts of the jaw during rapid growth in adolescence, resulting in a short prosthesis. Therefore, all the patients in our study were older than 16 years.
Outcomes
According to the radiographic findings, 90.6% (29/32) of the patients had sufficient bone volume for implant placement. This rate is comparable to the rates reported by Åbyholm et al. (1981) (91%) and Bergland et al. (1986) (90%) but superior to the rates reported by Collins et al. (1998) (86.86%), Amanat and Langdon (1991) (83%), and Nightingale et al. (2003) (71%) for patients with the mixed dentition. This result confirms the optimal outcomes of the proposed grafting technique.
All of the 29 patients had sufficient bone volume for implant placement, evaluated as Enermark score 1, which is considered successful bone grafting, representing more than 75% bone fill in the grafted site (not completely 100%). Therefore, the alveolar crest level of the tooth in the cleft usually appears to be 1 to 2 mm toward the apical compared with that of the contralateral tooth after secondary alveolar graft (Esper et al., 2009). To optimize the aesthetics of the prosthesis on implant, we did not perform implant placement at the alveolar crest level of the available bone because the crown on the implant subsequently would be too long. We followed the implant placement standard in the aesthetic zone in which the distance from the implant’s platform to the marginal gingival of the adjacent tooth is 3 mm. As a result, some implant threads are not covered by bone. Therefore, we decided to perform additional bone graft to cover all the exposed implant threads at the same time, with implant placement to support the soft tissue and improve the aesthetics of the prosthesis.
In additional bone graft, we used a mixture of autogenous particulate bone and Osteon (30% hydroxyapatite and 70% β-tricalcium phosphate) (Genoss, Suwon, Korea). We applied 2 different techniques in additional grafting based on the size of bone defect. Of the total 32 implants placed in our study, GBR grafting technique was indicated for 29 implants (90.6%) because of small defect size, whereas 3 implants (9.4%) that had greater bone defect required bone support around the crestal implant and was repaired with a bone ring technique (Stevens, 2010). The number of implants placed using the GBR technique was much higher compared with those using the bone ring technique. It proved that the bone defect after secondary bone grafting with the 2 iliac corticocancellous blocks was insignificant.
Dental implant placement in the alveolar cleft was considered to yield unsatisfactory results because of the low stability. However, some studies demonstrated the viability of this procedure (Cune et al., 2004; Kearns et al., 1997; Kramer et al., 2005). Our technique produced an implant survival rate of 100% in 29 patients with 32 implants during 3 years of follow-up. This result is in accordance with the findings of other studies: 98.6% in 47 patients with 71 implants observed for 1 to 5 years (Matsui, 2007), 98.4% in 120 patients with 123 implants over 1 year (de Barros Ferreira et al., 2010), 90% in 14 patients with 20 implants followed for 1 to 54 months (Kearns et al., 1997), and 96% in 15 patients with follow-up periods of 4 to 36 months (Härtel et al., 1999). Moreover, Takahashi (2011) demonstrated that implant placement maintains bone volume after secondary PCBM grafting. Endosteal dental implants placed in the grafted alveolus not only close the gap but also stimulate the bone during mastication (Dempf et al., 2002). However, oral health status, aesthetics, function, and patient desires should be considered before implant surgery. Long-term observation and comparison with results from other sites are required for a more definitive conclusion.
In this study, implants of 10 or 12 mm in length and 3.8 mm in diameter were used to ensure biomechanical stability. The implant length was determined from the alveolar height, which is frequently reduced in patients with clefts despite successful alveolar bone grafting. Esper et al. (2009) revealed irregularities of 1 to 2 mm in the soft tissue margin of the tooth adjacent to the cleft compared with the contralateral tooth. Therefore, an additional bone graft was placed during implant surgery to improve the soft tissue profile and aesthetics of the prosthesis. Guided bone regeneration was performed in most cases, and only 3 cases required the bone ring technique. This result again confirms the effectiveness of the grafting technique.
Primary implant stability is difficult to achieve in grafted bone. During implant surgery, several methods were applied to increase primary stability, such as undersized site preparation, bone compression, and use of rough tapered implants with more threads in the neck area, increasing the contact area between the bone and the implant. However, 12.4% of the implants alone were placed with an insertion torque greater than 35 N/cm2. According to Neugebauer et al. (2006), immediate loading in single-tooth indications should be considered only if the implant can be placed with an insertion torque over 35 N/cm2. Therefore, restorations were placed after a healing period of 6 months to achieve osseointegration.
Conclusions
The data indicate that the 2 iliac corticocancellous-block grafting technique is reliable to repair alveolar clefts and facilitates dental implant placement in patients with corrected CLP.
Author Note
This article was presented orally at the 8th Vietnam International Dental Exhibition and Congress, Ha Noi, Vietnam on August 19-21, 2015.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
Åbyholm, F, Bergland, O, Semb, G. Secondary bone grafting of alveolar clefts: a surgical/orthodontic treatment enabling a non-prosthodontic rehabilitation in cleft lip and palate patients. Scand J Plast Reconstr Surg. 1981;15:127–140.
Google Scholar | Crossref | Medline
________________________________________
Albrektsson, T . Repair of bone grafts: a vital microscopic and histological investigation in the rabbit. Scand J Plast Reconstr Surg. 1980;14:1–12.
Google Scholar | Crossref | Medline
________________________________________
Amanat, N, Langdon, JD. Secondary alveolar bone grafting in clefts of the lip and palate. J Craniomaxillofac Surg. 1991;19:7–14.
Google Scholar | Crossref | Medline | ISI
________________________________________
Amin, K, Stearns, Jeffrey W., Raymond, J. Secondary grafting in the alveolar cleft patient. Oral Maxillofacial Surg Clin N Am. 2002;14:477–490.
Google Scholar | Crossref | Medline
________________________________________
Bergland, O, Semb, G, Abyholm, FE. Elimination of the residual alveolar cleft by secondary bone grafting and subsequent orthodontic treatment. Cleft Palate J. 1986;23:175–205.
Google Scholar | Medline
________________________________________
Booij, A, Raghoebar, GM, Jansma, J, Kalka, WW, Vissink, A. Morbidity of chin bone transplants used for reconstructing alveolar defects in cleft patients. Cleft Palate Craniofac J. 2005;42:533–538.
Google Scholar | SAGE Journals | ISI
________________________________________
Bosshardt, DD, Schenk, RK. Biologic basis of bone regeneration. In: Buser, D , ed. 20 Years of Guided Bone Regeneration in Implant Dentistry. Hanover Park, IL: Quintessence Publishing; 2009:15–46.
Google Scholar
________________________________________
Boyne, P . Bone response to dental endosseous implants. In: Babbush, CA , ed. Dental Implants: Principles and Practice. Philadelphia, PA: WB Saunders; 1991:17–29.
Google Scholar
________________________________________
Boyne, PJ, Sand, NR. Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg. 1972;30:87–92.
Google Scholar | Medline
________________________________________
Bukhari, SGA, Pasha, B, Ahmed, W, Fazal, M, Jan, H. Alveolar bone grafting with mandibular symphysis as donor material. Pak Oral Dent J. 2009;29:3–8.
Google Scholar
________________________________________
Bureau, S, Penko, M, McFadden, L. Speech outcome after closure of oronasal fistulas with bone grafts. J Oral Maxillofac Surg. 2001;59:1408–1414.
Google Scholar | Crossref | Medline | ISI
________________________________________
Canady, JW, Zeitler, DP, Thompson, SA, Nicholas, CD. Suitability of the iliac crest as a site for harvest of autogenous bone grafts. Cleft Palate Craniofac J. 1993;30:579–581.
Google Scholar | SAGE Journals | ISI
________________________________________
Chen, YC, Chen, CH, Chen, PL, Huang, IY, Shen, YS, Chen, CM. Donor site morbidity after harvesting of proximal tibia bone. Head Neck. 2006;28:496–840.
Google Scholar | Crossref | Medline | ISI
________________________________________
Cho Lee, GY, García Díez, EM, Nunes, RA, Martí Pagès, C, Sieira Gil, R, Rivera Baró, A. Review of secondary alveolar cleft repair. Ann Maxillofac Surg. 2013;3:46–50.
Google Scholar | Crossref | Medline
________________________________________
Cohen, M, Figueroa, AA, Haviv, Y, Schafer, ME, Aduss, H. Iliac versus cranial bone for secondary grafting of residual alveolar clefts. Plast Reconstr Surg. 1991;87:423–427.
Google Scholar | Crossref | Medline | ISI
________________________________________
Collins, M, James, DR, Mars, M. Alveolar bone grafting: a review of 115 patients. Eur J Orthod. 1998;20:115–120.
Google Scholar | Crossref | Medline | ISI
________________________________________
Cune, MS, Meijer, GJ, Koole, R. Anterior tooth replacement with implants in grafted alveolar cleft sites: a case series. Clin Oral Implants Res. 2004;15:616–624.
Google Scholar | Crossref | Medline | ISI
________________________________________
de Barros Ferreira, S, Esper, LA, Sbrana, MC, Ribeiro, IW, de Almeida, AL. Survival of dental implants in the cleft area—a retrospective study. Cleft Palate Craniofac J. 2010;47:586–590.
Google Scholar | SAGE Journals | ISI
________________________________________
Dempf, R, Teltzrow, T, Kramer, FJ, Hausamen, JE. Alveolar bone grafting in patients with complete clefts: a comparative study between secondary and tertiary bone grafting. Cleft Palate Craniofac J. 2002;39:18–25.
Google Scholar | SAGE Journals | ISI
________________________________________
Denny, AD, Talisman, R, Bonawitz, SC. Secondary alveolar bone grafting using milled cranial bone graft: a retrospective study of a consecutive series of 100 patients. Cleft Palate Craniofac J. 1999;36:144–153.
Google Scholar | SAGE Journals | ISI
________________________________________
de Ruiter, A, Janssen, N, van Es, R, Frank, M, Meijer, G, Koole, R, Rosenberg, T. Micro-structured β-tricalcium phosphate for repair of the alveolar cleft in cleft lip and palate patients: a pilot study. Cleft Palate Craniofac J. 2015;52:336–340.
Google Scholar | SAGE Journals | ISI
________________________________________
Dickinson, BP, Ashley, RK, Wasson, KL, O’Hara, C, Gabbay, J, Heller, JB, Bradley, JP. Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg. 2008;121:209–217.
Google Scholar | Crossref | Medline | ISI
________________________________________
Enemark, H, Sindent-Pedersen, S, Bundgaard, M. Long-term results after secondary bone grafting of alveolar clefts. J Oral Maxillofac Surg. 1987;45:913–919.
Google Scholar | Crossref | Medline | ISI
________________________________________
Esper, LA, Sbrana, MC, Riberio, IW, de Siqueira, EN, de Almeida, AL. Esthetic analysis of gingival components of smile and degree of satisfaction in individuals with cleft lip and palate. Cleft Palate Craniofac J. 2009;46:381–387.
Google Scholar | SAGE Journals | ISI
________________________________________
Feichtinger, M, Mossböck, R, Kärcher, H. Assessment of bone resorption after secondary alveolar bone grafting using three-dimensional computed tomography: a three-year study. Cleft Palate Craniofac J. 2007;44:142–148.
Google Scholar | SAGE Journals | ISI
________________________________________
Goudy, S, Lott, D, Burton, R, Wheeler, J, Canady, J. Secondary alveolar bone grafting: outcomes, revisions, and new applications. Cleft Palate Craniofac J. 2009;46:610–612.
Google Scholar | SAGE Journals | ISI
________________________________________
Härtel, J, Pögl, C, Henkel, KO, Gundlach, KK. Dental implants in alveolar cleft patients: a retrospective study. J Craniomaxillofac Surg. 1999;27:354–357.
Google Scholar | Crossref | Medline | ISI
________________________________________
Ishii, M, Ishii, Y, Moriyama, T, Gunji, A, Morita, K, Imaizumi, F, Enomoto, S. Simultaneous cortex bone plate graft with particulate marrow and cancellous bone for reliable closure of palatal fistulae associated with cleft deformities. Cleft Palate Craniofac J. 2002;39:364–369.
Google Scholar | SAGE Journals | ISI
________________________________________
Kazemi, A, Stearns, JW, Fonseca, RJ. Secondary grafting in the alveolar cleft patient. Oral Maxillofac Surg Clin North Am. 2002;14:477–490.
Google Scholar | Crossref | Medline
________________________________________
Kearns, G, Perrott, DH, Sharma, A, Kaban, LB, Vargervik, K. Placement of endosseous implants in grafted alveolar clefts. Cleft Palate Craniofac J. 1997;34:520–525.
Google Scholar | SAGE Journals | ISI
________________________________________
Kindelan, JD, Nashed, RR, Bromige, MR. Radiographic assessment of secondary autogenous alveolar bone grafting in cleft lip and palate patients. Cleft Palate Craniofac J. 1997;34:195–198.
Google Scholar | SAGE Journals | ISI
________________________________________
Kline, RM, Wolfe, SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5–20.
Google Scholar | Crossref | Medline | ISI
________________________________________
Kramer, FJ, Baethge, C, Swennen, G, Bremer, B, Schwestka-Polly, R, Dempf, R. Dental implants in patients with orofacial clefts: a long-term follow-up study. Int J Oral Maxillofac Surg. 2005;34:715–721.
Google Scholar | Crossref | Medline | ISI
________________________________________
Lekholm, U . The use of osseointegrated implants in growing jaws, Int Oral Maxillofac Implants 1993;8:243–244.
Google Scholar | Medline
________________________________________
Long, RE, Semb, G, Shaw, W. Orthodontic treatment of the patient with complete clefts of lip, alveolus, and palate: lessons of the past 60 years. Cleft Palate Craniofac J. 2000;37:533.
Google Scholar | Crossref | ISI
________________________________________
Matsui, Y, Ohno, K, Nishimura, A, Shirota, T, Kim, S, Miyashita, H. Long-term study of dental implants placed into alveolar cleft sites. Cleft Palate Craniofac J. 2007;44:444–447.
Google Scholar | SAGE Journals | ISI
________________________________________
Mikoya, T, Inoue, N, Matsuzawa, Y, Totsuka, Y, Kajii, TS, Hirosawa, T. Monocortical mandibular bone grafting for reconstruction of alveolar cleft. Cleft Palate Craniofac J. 2010;47:454–468.
Google Scholar | SAGE Journals | ISI
________________________________________
Misch, E, Perel, ML, Wang, H-L, Sammartino, G, Galindo-Moreno, P, Trisi, P, Steigmann, M, Rebaudi, A, Palti, A, Pikos, MA. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5–15.
Google Scholar | Crossref | Medline | ISI
________________________________________
Murthy, AS, Lehman, JA. Evaluation of alveolar bone grafting: a survey of ACPA teams. Cleft Palate Craniofac J. 2005;42:99–101.
Google Scholar | SAGE Journals | ISI
________________________________________
Neugebauer, J, Traini, T, Thams, U, Piattelli, A, Zöller, JE. Peri-implant bone organization under immediate loading state. Circularly polarized light analyses: a minipig study. J Periodontol. 2006;77:152–160.
Google Scholar | Crossref | Medline | ISI
________________________________________
Nightingale, C, Witherow, H, Reid, FD, Edler, R. Comparative reproducibility of three methods of radiographic assessment of alveolar bone grafting. Eur J Orthod. 2003;25:35–41.
Google Scholar | Crossref | Medline | ISI
________________________________________
Peled, M, Aizenbud, D, Horwitz, J, Machtei, EE. Treatment of osseous cleft palate defects: a preliminary evaluation of novel treatment modalities. Cleft Palate Craniofac J. 2005;42:344–348.
Google Scholar | SAGE Journals | ISI
________________________________________
Posnick, JC, Ruiz, RL. Staging of cleft lip and palate reconstruction: infancy through adolescence. In: Wyszynski, DF , ed. Cleft Lip and Palate: From Origin to Treatment. New York, NY: Oxford University Press; 2002:319–353.
Google Scholar
________________________________________
Swan, MC, Goodacre, TE. Morbidity at iliac crest donor site following bone grafting of the cleft alveolus. Br J Oral Maxillofac Surg. 2006;44:129–133.
Google Scholar | Crossref | Medline | ISI
________________________________________
Stevens, MR, Emam, HA, Alaily, ME, Sharawy, M. Implant bone rings. One-stage three-dimensional bone transplant technique: a case report. J Oral Implantol. 2010;36:69–74.
Google Scholar | Crossref | Medline | ISI
________________________________________
Takahashi, T, Fukuda, M, Yamaguchi, T, Kochi, S. Placement of endosseous implants into bone-grafted alveolar clefts: assessment of bone bridge after autogenous particulate cancellous bone and marrow graft. Int J Oral Maxillofac Implants. 1999;14:86–93.
Google Scholar | Medline | ISI
________________________________________
Turvey, TA, Vig, K, Moriarty, J, Hoke, J. Delayed bone grafting in the cleft maxilla and palate: a retrospective multidisciplinary analysis. Am J Orthod. 1984;86:244–256.
Google Scholar | Crossref | Medline
________________________________________
Verdi, FJ, Lanzi, GL, Cohen, SG, Powell, R. Use of the Branemark implant in the cleft palate patient. Cleft Palate Craniofac J. 1991;28:305–307.
Google Scholar | Crossref | Medline | ISI